Laboratory of Organic Chemistry for Drug Development
Laboratory of Organic Chemistry for Drug Development
Japanese
Our laboratory is focused on advancing catalytic organic reaction through the development of new strategies for the synthesis of biologically active natural products and lead compound for drug discovery. The goal of our group is to contribute creative solutions to fundamental problems in chemistry that impact society. We are developing new catalysts and achieve practical total synthesis of natural products with the aim of drug development through technological innovation. It targets various functional molecules such as sugar chains and peptide chains.
Research Achievements
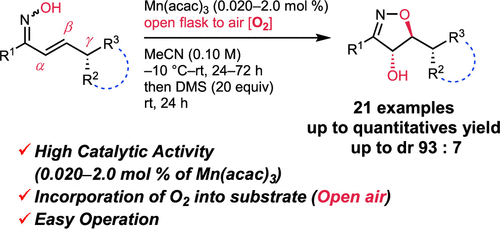
Manganese-Catalyzed 5-Endo-trig Oxygenative Cyclization of α,β-Unsaturated Oximes under Air and Ambient Conditions for the Synthesis of 4,5-Dihydroisoxazoles
J. Org. Chem. 2024, 89, 9, 6377–6388
Daisuke Yamamoto,* Daisuke Matsukawa, Ryusei Kikuchi, Yuki Narushima, Yuta Kumakura, Mana Ito, and Kazuishi Makino*
The stereoselective 5-endo-trig oxygenative cyclization of α,β-unsaturated oximes was achieved using molecular oxygen (O2) and a manganese catalyst. Several 4-hydroxy-4,5-dihydroisoxazoles were obtained in high yields by directly incorporating O2 from the atmosphere (eliminating the necessity for a pure oxygen environment) and using an unprecedentedly low loading of Mn(acac)3 (as little as 0.020 mol %) without additional additives. Because of its desirable features, such as operational simplicity, inexpensive catalyst, mild reaction conditions (open flask conditions at room temperature), and broad substrate compatibility, this novel reaction provides an attractive synthetic approach to producing 4-hydroxy-4,5-dihydroisoxazoles.
Stereoselective synthesis of the isoxazolidine ring via manganese(iii)-catalysed aminoperoxidation of unactivated alkenes using molecular oxygen in air under ambient conditions
Green. Chem. 2022, 24, 7162-7170.
Yamamoto, D.*; Hirano, I.; Narushima, Y.; Soga, M.; Ansai, H.; Makino, K.*
In this study, we developed a route for the tris(mono-ferrocene-functionalised β-diketonato) manganese(III)-complex catalysed diastereoselective oxygenative aminoperoxidation of unactivated alkenes using molecular oxygen in air. In the reaction, ethanol is used as a solvent, and it proceeds at room temperature under open air. Due to its wide range of substrate scope, functional tolerance, simple operation and mild and environmentally friendly conditions, the reaction is a promising synthetic approach for synthesising valuable isoxazolidine rings as not only privileged structures in natural products but also versatile synthons for 1,3-amino alcohols.
Direct Synthesis of N-Protected Serine- and Threonine-Derived Weinreb Amides via Diboronic Acid Anhydride-Catalyzed Dehydrative Amidation: Application to the Concise Synthesis of Garner’s Aldehyde
Shimada, N.*; Ohse,N.; Takahashi, N.; Urata, S.; Koshizuka, M. Makino, K.
Synlett (2021), 32(10),1024-1028.
Boronic Acid-Catalyzed Final-Stage Site-Selective Acylation for the Total Syntheses of O-3'-Acyl Bisabolol β-D-Fucopyranoside Natural Products and Their Analogues.
Nakamura, Y.; Ochiai, T.; Makino, K.; Shimada, N.*
Chemical & Pharmaceutical Bulletin (2021), 69(3), 281-285
Boronic Acid-Catalyzed Regioselective Koenigs-Knorr-Type Glycosylation
Shimada, N.*; Sugimoto, T.; Noguchi, M.; Ohira, C.; Kuwashima, Y.; Takahashi, N.; Sato, N.; Makino, K.
Journal of Organic Chemistry (2021), 86(8), 5973-5982
Synthesis of Weinreb amides using diboronic acid anhydride-catalyzed dehydrative amidation of carboxylic acids
Shimada, N.*; Takahashi, N.; Ohse, N.; Koshizuka, M.; Makino, K.
Chemical Communications (Cambridge, United Kingdom) (2020), 56(86), 13145-13148
Diboronic acid anhydride-catalyzed direct peptide bond formation enabled by hydroxy-directed dehydrative condensation
Koshizuka, M.; Makino, K.; Shimada, N.*
Organic Letters (2020), 22(21), 8658-8664
Total syntheses of seminolipid and its analogues by using 2,6-bis(trifluoromethyl)phenylboronic acid as protective reagent
Shimada, N.*; Fukuhara, K.; Urata, S.; Makino, K.*
Organic & Biomolecular Chemistry (2019), 17(31), 7325-7329